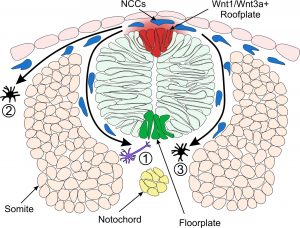
Model of Neural Crest Fate Specification in Zebrafish. Wnt signalling ligands such as Wnt1 and Wnt3a are expressed in the zebrafish dorsal neural tube and bias the fate of neural crest cells (NCCs). Cells that receive a low level of Wnt are biased towards a neuronal cell fate (1). Cells that are exposed to a high level of Wnt form pigment cells (2 & 3).
The neural crest is a population of cells that have the potential to develop into a remarkable number of different tissues. Neural crest cells are found in all vertebrate embryos, including humans, and form all body pigment cells in the skin, heart muscles, sensory neurons as well as bone and cartilage in the head and face. Understanding the biology of these specialised cells is essential, as neural crest development can sometimes go wrong in humans, causing physical abnormalities, including abnormal facial features and skin pigmentation, heart defects and tumours. Furthermore, many similarities have been drawn between the neural crest and cancer cells.
During their development, neural crest cells are exposed to molecular signals from surrounding cells that determine their fate. This review discusses the known role of one such molecular signal, known as Wnt, in the specification of neural crest cells.
The article outlines the key studies which have led to our current understanding, whereby neural crest cells that receive high levels of Wnt signal form pigmented skin cells as opposed to neurons. We have focused on studies conducted in the model organism zebrafish which is pioneering research on neural crest development. Finally we discuss the potential future directions of research in this field and the key questions which are yet to be answered.
Gemma Sutton, SWBio DTP student
Paper: Review: The Role of Wnt/β-Catenin Signalling in Neural Crest Development in Zebrafish by Gemma Sutton, Robert N. Kelsh and Steffen Scholpp in Frontiers in Cell and Developmental Biology.
